Introduction
Environmental concerns surrounding CO2 emissions and the use of fossil fuels in general has given greater impetus to the adoption of renewable fuel sources and technology. As a result, fuel cells have come a long way from being confined to high-tech laboratories to mass produced cars. The oxygen reduction reaction (ORR) forms a critical half cell in both fuel cells and other systems such as metal-air batteries. [1] Consequently, the development of efficient catalyst materials has received much attention. [2] This has lead to the synthesis and characterization of several suitable catalysts for ORR.
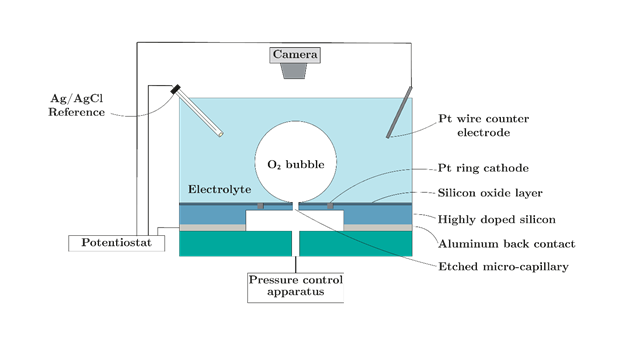
Figure 1: Schematic of proposed system
However, these material science driven studies overlook the importance of studying the behaviour and interaction between gas bubbles and the electrode surface. Recent studies have indicated that the gas-solid interface plays an important role in O2 bubble consumption kinetics. [1] The key challenge in understanding electrode kinetics for single bubbles lies in the application of advanced electrochemical techniques. Techniques such as EIS can yield a large wealth of information about electrochemical system. [3]–[5] However, such techniques are only applicable to systems that are stable in the order of tens of minutes and cannot be directly applied to single bubbles.
ASSIGNMENT
The primary objective of this project will be to study the influence of the three-phase contact line (TPCL) and the gas-bubble interfacial area (IA) on ORR. This will be achieved by using purpose-built electrodes with capillaries for bubble generation. The assignment can be broken down into the following stages:
1. Design and test a pressure control system that can generate and maintain the radius of a gas bubble from a capillary.
2. Use the pressure control system to study consumption kinetics of ORR by monitoring successive O2 bubbles at the capillary.
3. Conduct electrochemical experiments to characterize the system under stable conditions.
CONTACT
More information can be obtained from Akash Raman (a.raman@utwente.nl).
REFERENCES
[1] F. Chen et al., “Bubble Consumption Dynamics in Electrochemical Oxygen Reduction,” Chem. Res. Chin. Univ., vol. 36, no. 3, pp. 473–478, Jun. 2020, doi: 10.1007/s40242-020-0061-y.
[2] X. Wang et al., “Review of Metal Catalysts for Oxygen Reduction Reaction: From Nanoscale Engineering to Atomic Design,” Chem, vol. 5, no. 6, pp. 1486–1511, Jun. 2019, doi: 10.1016/j.chempr.2019.03.002.
[3] C. Gabrielli, F. Huet, and R. P. Nogueira, “Electrochemical impedance of H2-evolving Pt electrode under bubble-induced and forced convections in alkaline solutions,” Electrochimica Acta, vol. 47, no. 13–14, pp. 2043–2048, May 2002, doi: 10.1016/S0013-4686(02)00070-1.
[4] J. C. Garcia-Navarro, M. Schulze, and K. A. Friedrich, “Measuring and modeling mass transport losses in proton exchange membrane water electrolyzers using electrochemical impedance spectroscopy,” Journal of Power Sources, vol. 431, pp. 189–204, Aug. 2019, doi: 10.1016/j.jpowsour.2019.05.027.
[5] B. Chmielowiec et al., “Experimental Measurement of Overpotential Sources during Anodic Gas Evolution in Aqueous and Molten Salt Systems,” J. Electrochem. Soc., vol. 166, no. 10, pp. E323–E329, 2019, doi: 10.1149/2.1001910jes.
