The field of HTA aims to quantitatively evaluate the (potential) value of a wide range of health innovations across disease areas and applications. Examples of such innovations are basic aids in chronic care, surgical robots, e-Health apps, 3D printing, telemonitoring, as well as many innovative imaging technologies which are the focus of CMI-NEN.
In the HTA domain, a holistic view on development and evaluation of technologies is taken, starting from conceptualization through technical feasibility and up to actual (clinical) impact, health benefits, cost savings, and implementation. Already during technology development iterative (and increasingly accurate) early HTA can provide valuable insights informing decisions on further development and potential applications. HTA is multidisciplinary by nature, given the broad range of related aspects covered and typically involves collaborations between technology developers/manufacturers, medical specialists, care providers, health economists, epidemiologists, and data scientists.
HTA of diagnostic innovations is a key domain of CMI-NEN and is quite challenging, as benefits from such innovations are generally indirect, that is, do not result directly from testing but from downstream consequences such as improved patient management decisions. However, diagnostic innovations may potentially also have broader impact, with benefits for citizens, patients, care providers, or society as a whole, as illustrated in the figure below.
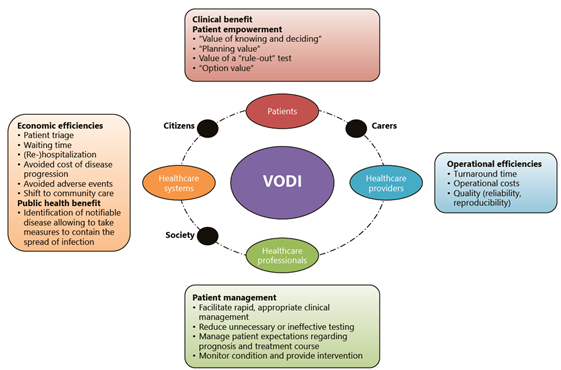
Wurcel V. Wurcel, et al. The Value of Diagnostic Information in Personalised Healthcare: A Comprehensive Concept to Facilitate Bringing This Technology into Healthcare Systems. Public Health Genomics. 2019;22(1-2):8-15.
Challenges
Current more specific and additional challenges regarding the (early) HTA of diagnostic innovations include, for example
- Evaluation of increasingly complex and personalized diagnostic pathways
- Assessment of the impact of multiple combined test results on (clinical) decision making
- Evaluation of the impact and added value of companion diagnostics
- Reflecting heterogeneity in test outcomes over time (for example in tumour genetic mutations)
- Accounting for the value of non-health and non-monetary outcomes benefits from testing (for example, the ‘value of knowing’ if a disease is present) using new value assessment frameworks
Within CMI-NEN, we address such challenges in the context of concrete (clinical) projects focusing on assessing the added value and optimal use of new diagnostic technologies.
HTA projects
B3CARE
The UMCG, UT and Siemens collaborate in the B3CARE project to enable early detection and prevention of lung cancer, chronic obstructive pulmonary disease (COPD) and cardiovascular disease (CVD), the so-called Big-3 (B3) diseases. These B3 diseases are expected to cause most deaths by 2050. Within this project, innovative low-dose computed tomography (CT) allows simultaneous, integrated assessment of early imaging biomarkers of lung cancer, COPD and CVD which may inform treatment decisions in order to lower the disease burden.
B3CARE aims to advance the technology readiness level (TRL) of integrated B3 screening with at least 2 levels by developing a large, high-quality imaging data biobank to provide biomarker reference values and validate B3 biomarkers using novel image analysis software and novel machine learning approaches. Also, the expected substantial health and economic benefits of combined B3 imaging biomarkers for personalized health strategies are evaluated.
More information
For project details and results see the website of B3CARE





