Organization:
Funded by: | UT/DFG |
PhD: | |
Supervisor: | |
Daily Supervisor: | |
Collaboration: | This project is part of : |
Description:
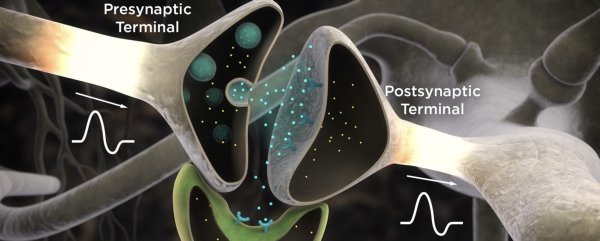
Neurons transfer information mainly through connections called the tripartite synapse, which involves a pre-synaptic and post-synaptic neuron and an astrocyte. Reliable generation of action potentials and synaptic transmission is essential for normal brain function, such as attention, motor control, learning, or memory storage and retrieval. During ischemia, e.g., during a stroke, the blood flow and oxygen supply are restricted. Insufficient energy supply quickly results in synaptic transmission failure. Subsequently, prolonged or more severe energy depletion causes loss of membrane potentials, cell swelling, and, ultimately, cell death. Previous computational modelling has shown a tipping point beyond which recovery is impossible without intervention [1,2]. Therefore, it is important to study what happens during energy deprivation and how further damage, such as cell death, can be prevented.
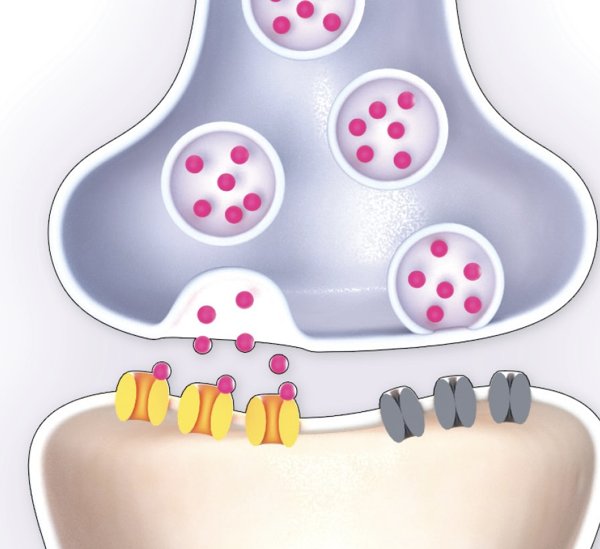
Synaptic transmission critically depends on the opening and closing of various voltage- and ligand-gated ion channels and the associated ionic currents. To maintain ion homeostasis, transmembrane ion fluxes are counteracted by energy-dependent ion transporters, such as the sodium-potassium ATPase. Synaptic transmission further includes vesicle transport (endo- and exocytosis) and recycling of neurotransmitters, which is also dependent on ATP. The current model, by Manu Kalia [1], consists of a pre-synaptic neuron, an astrocyte and the extracellular space.
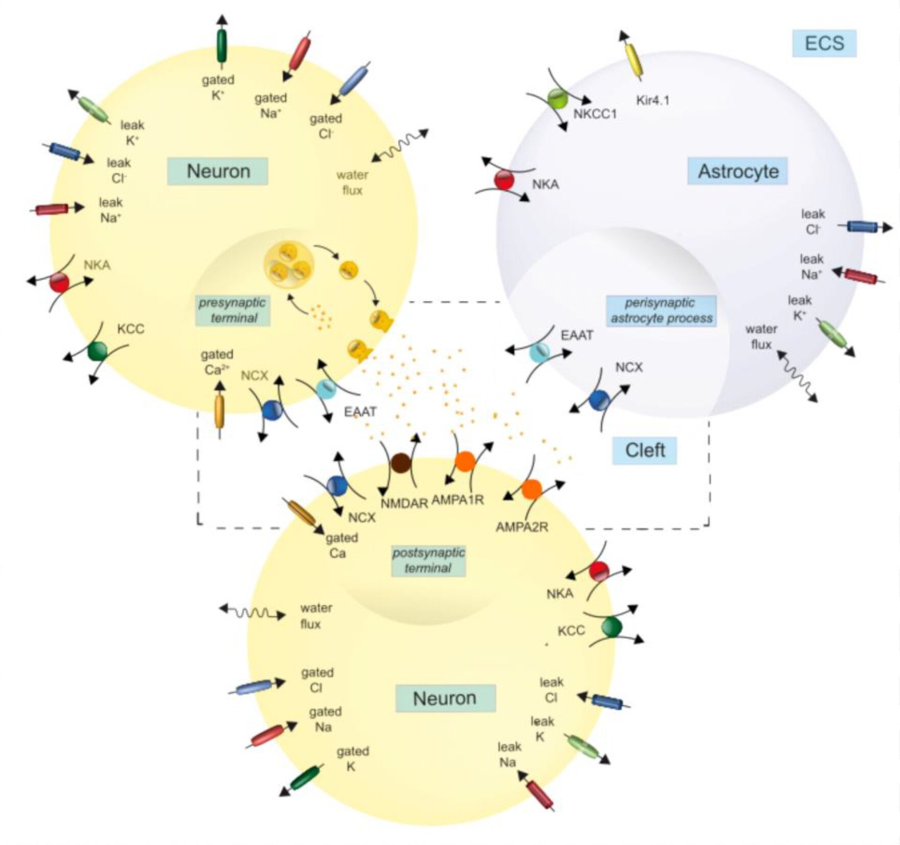
The project's goal is to model the tripartite synapse during metabolic stress by extending the current model with a post-synaptic neuron, pH dependency and volume dynamics. The extension will be calibrated to better reproduce and understand experimental observations during energy depletion. With these multiple energy-dependent processes, we can study which processes are most affected during ischemia. Furthermore, we can explore critical values for (ir)reversibility and recovery speeds.
The extension includes sodium, potassium, chloride and calcium ion channels and the associated leak currents. Furthermore, it contains ion transporters, energy-dependent endo- and exocytosis and AMPA and NMDA receptors. The figure shows the ion currents and transporters the model will include. ATP-dependent neurotransmitter recycling could also be relevant.
After the single-cell model is finished, we want to understand energy-dependent processes for multiple synaptic connections. The idea is to study the effect of energy deprivation on small populations through ion-based neural mass models and to simulate EEG rhythms. Such EEG rhythms may be used to interpret the level of ischemia.
[1] Kalia, M. (2022). Data, models and transitions in computational neuroscience: Bottom-up and top-down approaches [PhD Thesis - University of Twente]. doi: 10.3990/1.9789036554091
[2] Koen Dijkstra, Jeannette Hofmeijer, Stephan A. van Gils, Michel J.A.M. van Putten, A Biophysical Model for Cytotoxic Cell Swelling, J Neurosci. 2016 Nov 23; 36(47): 11881–11890.
doi: 10.1523/JNEUROSCI.1934-16.2016
Pictures: